B. infantis probiotic for infants: A breakthrough in infant care, so you can help babies thrive
Try Evivo for your patients today; a superior B. infantis probiotic for infants
Parents want your recommendations
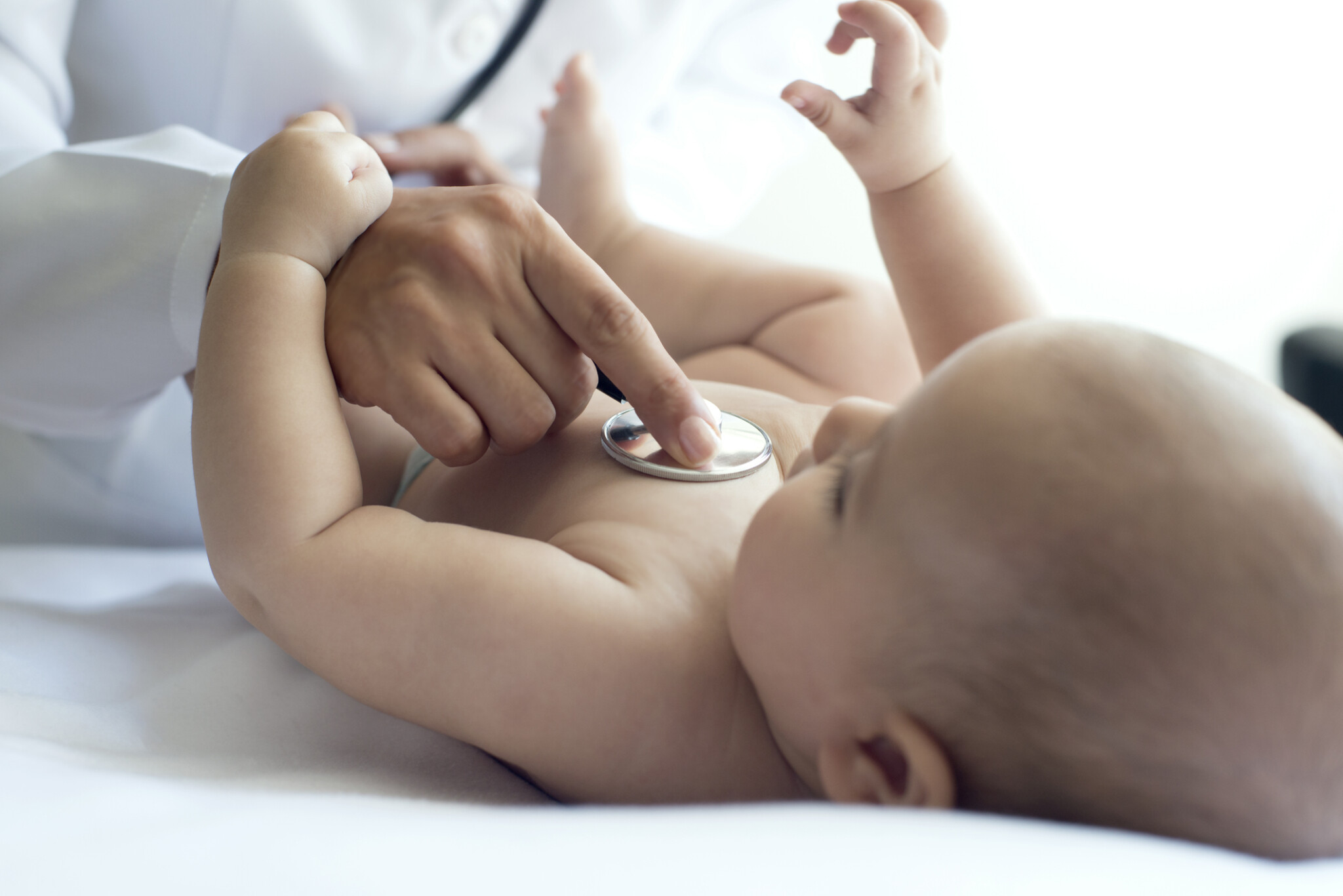
Whether you are a pediatrician, lactation consultant, OB, or midwife, when you recommend Evivo’s bifidobacterium infantis probiotic, you’re helping baby meet a key milestone that safeguards health – establishing a healthy gut microbiome.
Evivo Growth Partners
Join healthcare professionals, parents and others to share your passion for Evivo probiotics with B. infantis EVC001. We’ve created three options to help you get started quickly – choose what works best for you and your community.
For Health Systems
Evivo focuses on restoring the good bacteria B. infantis EVC001 to the infant gut microbiome to support gut and immune system function. Raise the standard of care at your place of practice with Evivo.
Resources
We can explain the science behind Evivo because we made the discoveries – explore our peer-reviewed articles, white papers, podcasts, and more.
Evivo Products
Our research to understand mysteries within breast milk led us to discover the power of its synergy with B. infantis EVC001. The single strain that baby needs, and only found in Evivo. Evivo is intended for use in term infants.
Evivo Drops

A daily probiotic for newborns fed any amount of breast milk (or HMOs found in formula) to help establish a protective gut microbiome and support healthy immune function.
**Starting with orders placed on July 2nd and after, Evivo Drops will include Olive Oil instead of Sunflower Oil, but same great Evivo and Organic Vitamin D3 for healthy bones & teeth.**
Schedule a presentation with our team

Carrie McGuckin, BSN, RNC
NICU Nurse
Director, Corporate Excellence

Mandy Kennedy
Chief Marketing Officer
Read bioConnect with us
Email us at customerservice@evivo.com for customer service, support, and to learn more about our probiotics.